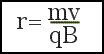
Operation of a mass spectrometer.
Lorentz force.
Radial (centripetal) force
Pre-requisite skills: Elementary understanding of basic electrostatics and Newton's Second Law.
Approximate completion time: Under an hour.
Provide sufficient detail to verify that the assignment was completed in a meaningful manner.
MERLOT site: Mass Spectrometer, by Angel Franco Garcia. (direct link).
Applet by Angel Franco Garcia
Hydrogen
1. Familiarize yourself with the operation of the applet. On the lower left there is a menu for selecting a particular atom and some of its isotopes. The atoms here are assumed to be net postively charged because they each have been stripped of one electron, leaving more protons than electrons. We will start this assignment with hydrogen (Hidrogeno).
(a) Press the trajectory (trayectoria) button. The lines that appear show the paths each atom (and its isotopes) take when traveling through the magnetic field. Judging by the curvature of these lines, which direction is the magnetic field pointing?
(b) Using Newton's Second Law, derive the following expression for the radius of curvature of each ion in terms of the field strength B and the velocity v, charge, q, and mass m of the ion.
2. On the lower right of the applet is a text field for controlling the external magnetic field, B, measured in gauss (10,000 gauss equals 1 tesla). According to the expression above, the radius of curvature r should decrease as the field strength B is increased. Explain why this is expected in terms of the net force acting on the particle. Does the applet verify your prediction?
On the right side of the applet there is a text field for controlling the electric field strength, E. (If you are interested in understanding the purpose of this electric field, see The Mass Spectrometer Page.) In a nutshell, the electric field, in conjunction with the magnetic field, selects ions of a particular velocity that will pass into the semicircular region.
Carbon
3. (challenging) If not done so already, set the electric field E equal to 12.0 N/C and the magnetic field equal to 2 gauss. The ions will reach a speed of v = E/B just before they enter the semicircular area. Now select carbon (Carbono) from the menu on the lower left and press the Trajectory button. Adjust the magnetic field until the ions reach a measureable radius of curvature. By measuring the radii of curvature (using the scale) which isotopes of carbon appear? Press the Results (Respuesta) button to verify your answer.
4. (Essay) Describe the importance of the mass spectrometer in scientific research. As one example, why would it be important to separate the isotopes of carbon?
Helpful Resources
- Mass Spectrometer by Tony Abou-Assaleh..
- Mass Spectrometer by Larry Gladney.
- Charged ion Motion in E/M Field by Fu Kwun Hwang.
- Motion in an Electromagnetic Field by Mark Sutherland.
- Charged ions Moving in a Magnetic Field by Sergey Kiselev
Return to Web Assignments